Step 1

Stained cells are encapsulated within water droplets using a droplet generator before being directed to a bifurcation droplet trap. The droplets then travel through the system via a syringe pump, with some being intercepted by traps. Trapped droplets proceed through the Microfluidic chip, where they are rotated by a constant oil flow. This rotation facilitates thorough imaging of cells from multiple angles, enhancing analytical perspectives.
Step 2
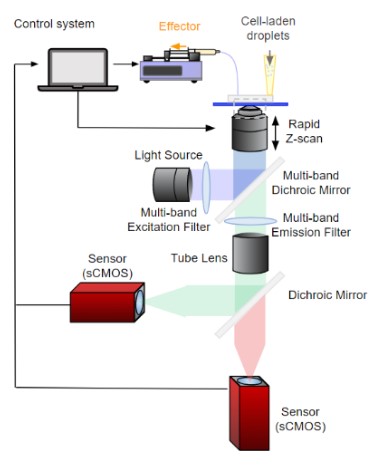
Our optical setup integrates two cameras and a series of optical filters, ensuring each camera receives a distinct fluorescent signal. When showcasing the video of cell rotation, one camera focuses on the blue-fluorescing nucleus while the other captures the red-fluorescing extracellular component. Following camera setting adjustments, simultaneous recording on both cameras commences, with the activation of one triggering the other to start recording.
Step 3
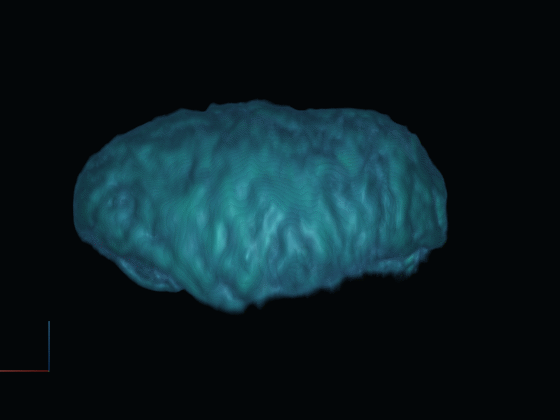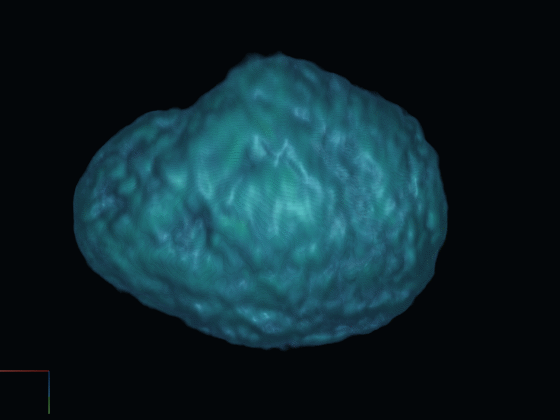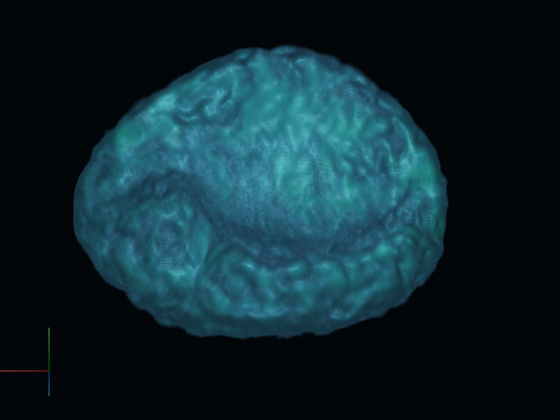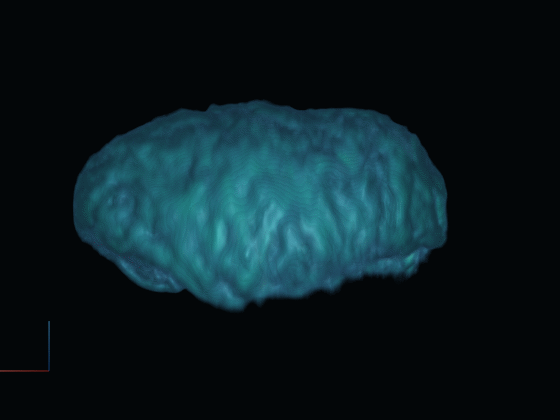
After gathering 2D projections of the rotating cell, we generate a 3D reconstruction of the cell. This reconstructed model offers insights into the cell's surface features and internal structures such as the nucleus, using distinct fluorescent dyes.